When Bonds Break: A "Chemical Bonding" Story-lined Unit
- Zip
What educators are saying
Also included in
- This BUNDLE consists of a year's worth of Chemistry Problem-Based Learning Units. They are also sold separately on TPT.All of the units have two sets of files. The "Classroom" files should be used in an in-person classroom setting. The "Absent" files can be used for long-term distance learning, homePrice $76.10Original Price $85.80Save $9.70
Description
When Bonds Break: A Problem-Based "Chemical Bonding" Unit for High School Chemistry (PBL)
Story:
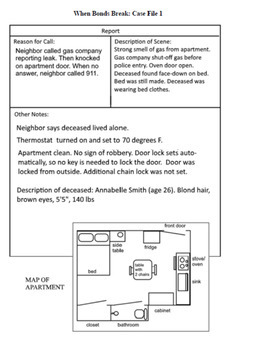
A woman is found dead in her apartment and all signs point to accidental carbon monoxide poisoning. But is the case really that simple? Students solve the case while learning about ionic, covalent, and metallic bonds.
Please see the preview for a list of materials and first day activities.
This product is also part of a BUNDLE found here.
Objectives:
General
- Construct models to express the arrangement of electrons in atoms of representative elements using electron configurations and Lewis dot structures.
- Construct an argument to support how periodic trends such as electronegativity can predict bonding between elements.
- Name and write the chemical formulas for ionic and covalent compounds using International Union of Pure and Applied Chemistry (IUPAC) nomenclature rules.
- Classify and draw electron dot structures for molecules with linear, bent, trigonal planar, trigonal pyramidal, and tetrahedral molecular geometries as explained by Valence Shell Electron Pair Repulsion (VSEPR) theory.
- Analyze the properties of ionic, covalent, and metallic substances in terms of intramolecular and intermolecular forces.
NGSS
HS-PS1-2. Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
HS-PS1-3. Conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles
Outline:
Day 1:
- Introduction
- What’s in a Letter? Lab
- Case File 1
Day 2:
- Why Does Carbon Monoxide Kill?
- Case File 2
Day 3:
- Types of Bonds Notes
- Types of Bonds Practice
- Case File 3
Day 4:
- Types of Bonds Review Activity
- Drawing Lewis Dots for Covalent Bonds Notes
Day 5:
- Naming Covalent Molecules
- Covalent Bonds Practice WS
- Case File 4
Day 6:
- Polar Covalent Bonds Notes
Day 7:
- Intermolecular Forces
- Like Dissolves Like Practice
Day 8:
- The Shape of Molecules (VSEPR)
- Case File 5
Day 9:
- Types of Bonds and Physical Properties
- Physical Properties and Bond Type Lab
Day 10:
- Drawing Lewis Dots for Ionic Bonds Notes
- Case File 6
Day 11:
- Writing Ionic Formulas with Oxidation Numbers
- Oxidation Numbers Matter
Day 12:
- Polyatomic Ions
- Case File 7
Day 13:
- Metallic Bonds Notes
- Making an Alloy Lab (optional)
- Mixed Bonding WS
Day 14:
- Activity Series Lab
- Using an Activity Series
- Bonding Review
Day 15:
- Testing the Earring Lab
- Conclusions/ Report
Copyright © E. Stubbe (The Wasp Whisperer)
All rights reserved by author.
Terms of Use: This document is for personal use only and may only be used by the original purchaser. This entire document, or any parts within, may not be reproduced or displayed for public viewing. You may NOT electronically post this product online including to teacher blogs, classroom websites or school networks. Failure to comply is a copyright infringement and a violation of the Digital Millennium Copyright Act (DMCA).