Turkey Towers Orbital Notation and Electron Configuration Inquiry Interactive
- PPTX
Description
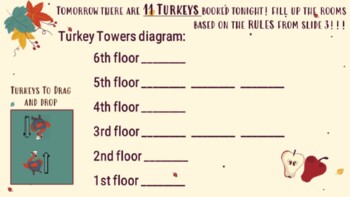
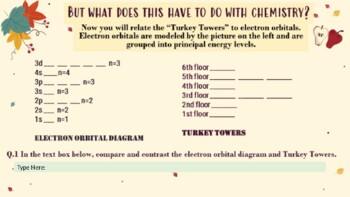
Are you looking for a fall-themed slides-based interactive to help your chemistry students ease into Orbital Notation and Electron Configuration. Inspired by the classic Hog Hilton simulation, this inquiry-based activity introduces the principles that govern orbital notation and electron configuration in a fun, drag and drop and fill-in style interactive.
Topics Covered:
Aufbau Principle
Hunds Rule
Pauli's Exclusion Principle
This digital lab uses a free design template from
https://slidesgo.com/theme/happy-thanksgiving
The contents and layout of this activity is entirely original otherwise and should be treated as the property of the poster. Please do not distribute, sell or otherwise misrepresent the author of this work. Please refer educators who would like to use this product to download directly from this Teachers Pay Teachers listing. Thank you!