AP Chemistry 9.1 Intro to Entropy Guided Inquiry Investigation
Kathleen Regovich
0 Followers
Resource Type
Standards
NGSSHS-PS3-4
Formats Included
- Google Docs™
- Excel Spreadsheets
Kathleen Regovich
0 Followers

Made for Google Drive™
This resource can be used by students on Google Drive or Google Classroom. To access this resource, you’ll need to allow TPT to add it to your Google Drive. See our FAQ and Privacy Policy for more information.
Also included in
- This is a worksheet, spreadsheet, and teacher guide for AP Chemistry standard 9.1 Intro to Entropy. Students use the statistical definition of entropy as the number of microstates that correspond to a particular macrostate. Students use several different statistical models to draw conclusions aboutPrice $5.00Original Price $6.00Save $1.00
Description
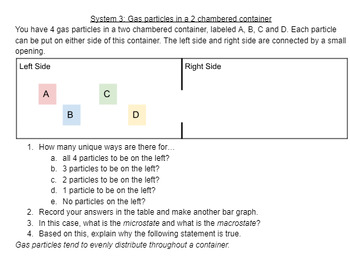
This is a guided inquiry investigation for AP Chemistry standard 9.1 Intro to Entropy. Students use the statistical definition of entropy as the number of microstates that correspond to a particular macrostate. Students use several different statistical models to draw conclusions about the relationship between entropy, statistics, temperature, diffusion, and states of matter. An accompanying spreadsheet template is linked in the document. Students may need pennies and dice for this activity.
Total Pages
Answer Key
Not Included
Teaching Duration
N/A
Report this resource to TPT
Reported resources will be reviewed by our team. Report this resource to let us know if this resource violates TPT’s content guidelines.
Standards
to see state-specific standards (only available in the US).
NGSSHS-PS3-4
Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics). Emphasis is on analyzing data from student investigations and using mathematical thinking to describe the energy changes both quantitatively and conceptually. Examples of investigations could include mixing liquids at different initial temperatures or adding objects at different temperatures to water. Assessment is limited to investigations based on materials and tools provided to students.