468 Task Cards & 5 Mastery Mats~BIG CHEMISTRY BUNDLE~16 Complete Sets
- Zip
What educators are saying
Description
SAVE 33% on this Big, Year Long Bundle that contains 16 complete sets of Chemistry Task Cards covering: (1) the atom, (2) acids/bases/pH, (3) solutions, (4) the mole, (5) thermochemistry, (6) gas laws, (7) oxidation-reduction, (8) empirical and molecular formulas, (9) stoichiometry, and (10) ionic and covalent bonding, (11) conversions & measurement, (12) chemical reactions, (13) electron configurations, (14) the periodic table, (15) writing chemical equations from word equations, and (16) matter & energy. The Writing Chemical Equations, Empirical Formulas, and Stoichiometry sets also include detailed printable placemats. There are 468 Task Cards in this 139 page bundle.
⭐⭐⭐ DISTANCE LEARNING USE ⭐⭐⭐
This bundle contains a separate PDF file of each task card set for each topic. You can easily email or assign the pdf file for a single topic via Google Classroom or Google Drive. Students would then return their answers via a Google Doc that they Create.
NOTE: Some cards in certain topics will require drawing or complex notations (for example: electron dot diagrams/electron configurations/ion symbols) that can not easily be created digitally by your students.
⭐⭐⭐ INCLUDES A POWERPOINT TEMPLATE TO ADD YOUR OWN CUSTOM TASK CARDS ⭐⭐⭐
Title, Question, and Card # can be edited in PowerPoint. Save as a PDF and print.
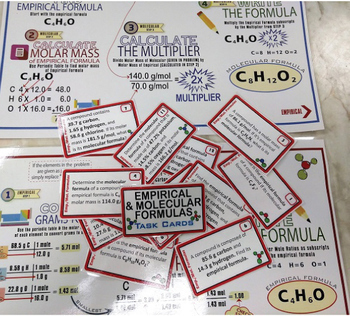
Each Task Card set has a unique border color and icon making for better organizing and use in centers. Each set includes a custom Student Answer Sheet and Teacher Answer Key. Sets 8, 9, and 15 also include easy to follow, step-by-step "Placemats" to help students master these multi-step, challenging topics.
All Task Cards and mats can be printed on Regular, Letter Sized paper. I recommend using Adobe Reader (which is a free download) for printing.
3 Task Card Sets in this Bundle include Mastery Mats:
*Writing Chemical Equations
*Empirical Formulas
*Stoichiometry
3 Size Options for Printing Mastery Mats
2 Sided Handout:
1) LETTER (regular copy paper) (8 1/2" x 11")
Wider Placemat Sized Options:
2) LEGAL(8 1/2" x 14")
3) LEDGER (11" x 17")
The cards and mastery mats can be printed in color or black and white and work best on card stock and can be laminated for years and years of use.
This bundle currently includes:
(2) Acids, Bases & pH Task Cards
(5) Thermochemistry Task Cards
(6) Gases & Gas Laws Task Cards
(7) Oxidation-Reduction Task Cards
(8) Empirical & Molecular Formulas Mats & Task Cards
(9) Stoichiometry Mats & Task Cards
(10) Ionic & Covalent Bonding Task Cards
(11) Accuracy & Measurement Task Cards
(12) Chemical Reactions (Balancing Equations & Types of Reactions)Task Cards
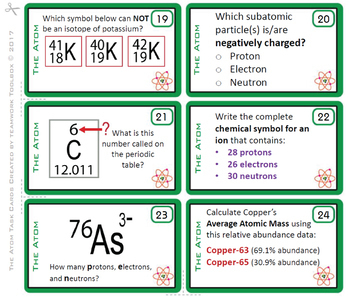
(13) Electron Configurations Task Cards
(14) The Periodic Table Task Cards
(15) Writing Chemical Equations Mastery Mat & Task Cards
(16) Matter & Energy Task Cards
-Included PowerPoint Template that allows you to Add Your Own Custom Task Cards.
The Title, Question, and Card # can be Revised.
***If new sets are added, the bundle price will increase. Purchase now and download any future additions at no additional charge!