10 Google Form Quizzes: Density (100 Questions and Self Grading!!)
- Google Forms™

Products in this Bundle (10)
showing 1-5 of 10 products
Bonus
Description
Are you looking for a unique and effective way to enhance your students' learning experience in the classroom?
I have created 10 quizzes that cover the topic of DENSITY and are specifically designed to engage and challenge your students. However, when creating these quizzes on Google Forms, I encountered a minor issue with subscripts and superscripts not being supported. I was able to create these quizzes in a way that will allow your students to fully understand the concepts being taught. By incorporating these quizzes into your curriculum, you can provide your students with a fun and interactive way to learn about DENSITY.
Below are some of the vocabulary and concepts I use in the quizzes.
Vocabulary/Concepts
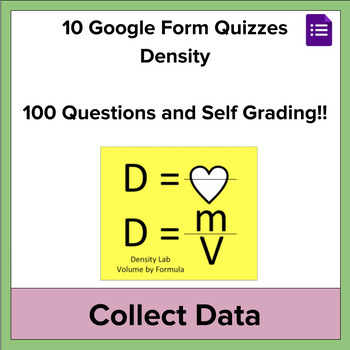
- Density = Mass/Volume
- Mass
- Volume
- Density
- Sink or float
- Calculations
- True and false questions
Each of them are ten questions. They are intended as a review or a warm up. They are self grading and the students can easily see their responses. It makes a great formative assessment at the start of class or used as a review before an assessment. QUIZ
FEATURES
- 10-question quizzes
- All questions are multiple-choice
- Makes a quiz, warmup, or exit ticket
DEFAULT QUIZ SETTINGS
- Collects student's email address
- Shuffles the order of the questions
- Shuffles the order of the answers in each question
- Students are immediately emailed with their results
- Each questions is worth 1 point
- All questions are required to be answered
- All these settings are customizable for your teaching style
AFTER PURCHASING
- All google forms will be added to your google drive. You will have owner rights to the form. You will be able to change, add, or delete questions.
How to share or assign to your students.
- Click the “share button”
- Copy the link from the new window
- Email the link, hyperlink it in a document on Google Classroom
- Students can click on the link to access the form.
Once students complete the quiz
- Quiz will be auto-graded
- A .csv file will be created that breaks down the results of your students
- The .csv can be opened as a Google Sheet in the Google Suite.
This product is editable. You can easily add questions, delete questions or change any of the questions.
Join my Email List to get exclusive FREE content!
Thank you for purchasing the quizzes. I have tried to make it user friendly and ready to go. Just share it with your students and get immediate feedback, so you can focus more of your energy on teaching. I hope you enjoy the product.
Thank you!